Since Sony introduced the first commercial lithium-ion battery , graphite materials have been the mainstream of anode materials. The graphite anode has a rapid decrease in potential after lithium insertion, and is close to the potential of the metal Li anode. This characteristic is good in that it can increase the voltage of the lithium ion battery and increase the energy density of the lithium ion battery. The bad side is too low. The potential causes decomposition of the carbonate-based organic electrolyte, thereby forming a passivation layer: a solid electrolyte membrane, which is what we usually call an SEI membrane.
Generally, we believe that SEI is the key to protect the negative electrode and electrolyte. The SEI film can avoid direct contact between the electrolyte and the negative electrode, reduce the decomposition of the electrolyte, and also avoid the co-embedding of Li+ with solvent molecules, causing the expansion of the graphite negative electrode, resulting in capacity decline. drop. Therefore, the majority of electrolyte manufacturers in the process of electrolyte design must first consider how to form a more stable SEI film, so various additives that help to improve the film quality of SEI film emerge in an endless stream, such as our common VC, FEC Etc., are commonly used SEI film forming additives.
But recently, from the study of King Abdullah University of Science and Technology (this is a super rich university, interested to know), JunMing et al. found that the role of SEI film in lithium-ion batteries is not What we imagine is important.
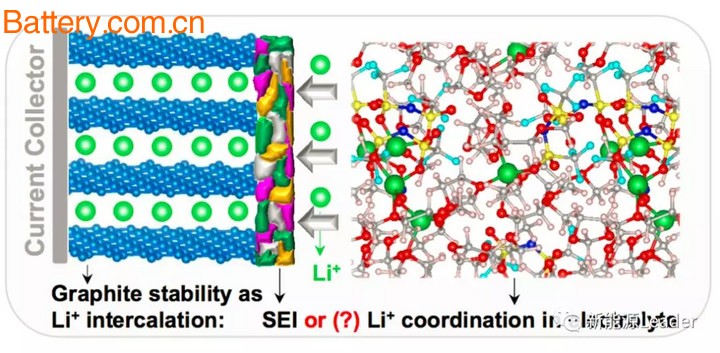
Most of the solvents used in commercial lithium-ion battery electrolytes have the problem of co-embedding of Li+-solvent, especially PC solvent. When co-embedding occurs, the structure of graphite will be seriously damaged, resulting in delamination of graphite, which seriously affects the graphite negative electrode. Cycle performance. In general, we believe that the SEI film of the negative electrode plays a key role in preventing solvent co-intercalation, but the study by Jun Ming et al. found that increasing the concentration and addition of electrolyte lithium salt (LiTFSI) compared to the SEI film. The inorganic additive (LiNO3) plays a greater role in preventing solvent co-intercalation.
Why do we say this? Let’s first look at the experiment by Jun Ming et al. First, Jun Ming assembled a graphite/metal Li half-cell and then injected a commercial electrolyte (LiPF6, 1 mol/L, EC:DMC=1:1). During the first discharge, as the Li is embedded, the potential of the negative electrode is lowered, and decomposition of the electrolyte and formation of the SEI film occur.
In the subsequent cycle, due to the formation of the SEI film, the cycle curve of the battery became very smooth, indicating that the SEI film can provide protection. However, when Jun Ming took out the above-mentioned negative electrode that had formed the SEI film, reloaded it into the half-cell and added a new ether electrolyte (1.0M/0.4M electrolyte, ie LITFSI, 1M, LiNO3, 0.4M, DOL/DME=1:1, a commonly used electrolyte in Li-S batteries, it was found that a large irreversible capacity was still generated during the first discharge/charge process, mainly due to the decomposition of the electrolyte on the surface of the negative electrode. Solvent co-intercalation, but if we increase the lithium salt concentration of the above electrolyte to 2.5M (2.5M / 0.4M electrolyte LITFSI, 2.5M, LiNO3, 0.4M, DOL / DME = 1:1 After that, the decomposition of the electrolyte can be remarkably suppressed (as shown in the following figure c).
Then Jun Ming will form the negative electrode of the SEI film in the ether 2.5M/0.4M electrolyte, and put it back into the low concentration ether 1.0M/0.4M electrolyte, and the electrolyte will start on the negative electrode surface. A significant decomposition occurs (as shown in Figure d below). This indicates that 1) the SEI film formed in the carbonate electrolyte solution is not very effective in avoiding delamination and spalling of graphite; 2) increasing the concentration of the lithium salt can effectively suppress the failure of the graphite negative electrode.

So what is the reason for the high concentration of LiTFSI electrolyte to significantly improve the stability of the graphite anode? To this end, Jun Ming was configured with different LiTFSI concentrations and formulations of electrolytes, and the results are shown below. As can be seen from the following figure d, with the increase of LiTFSI concentration (from 1M to 10M), the stability of the electrolyte has been significantly improved (10M concentration electrolyte performance degradation is mainly due to excessive concentration, resulting in viscosity increase and Ion migration is difficult), and the addition of LiNO3 to the electrolyte further enhances the stability of the electrolyte.
XRD studies have shown that graphite can reversibly intercalate and desorb Li+ in a higher concentration of 2.5M/0.4M electrolyte, but in a lower concentration of 1.0M/0.4M electrolyte, due to the presence of graphite in the solvent The delamination occurs under the action of co-embedding, resulting in the irreversibility of the insertion of Li+.

In order to study the mechanism of the above phenomenon, Jun Ming used Raman spectroscopy to study the solution structure of the electrolyte. The study shows that the TFSI- ions in the electrolyte exist in several different forms according to the strength of interaction with Li+: free Ion FI, loose ion pair LIP, tight ion pair IIP and polymer ion pair AIP.
The experimental data show that: 1) At lower concentrations, the main form of TFSI- is FI or LIP. When the lithium salt concentration is increased, TFSI- will change to LIP and IIP; 2) DME can be more than DOL Good dissolution of the lithium salt makes TFSI-more present in the form of FI; 3) NO3- can significantly enhance the interaction between Li+-TFSI-.
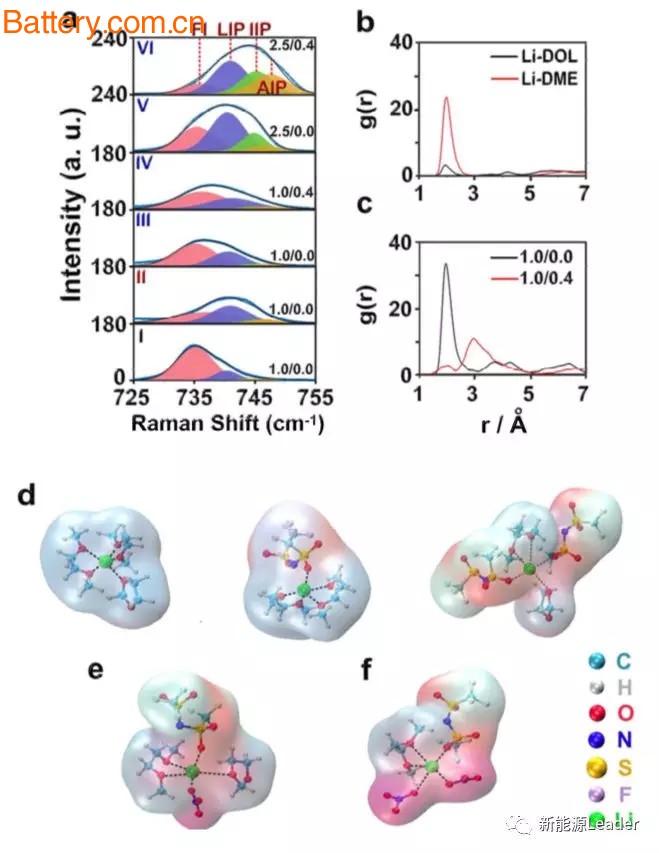
In general, we believe that Li+ will form a solvated shell that will contain anion and solvent molecules. Jun Ming used the density function theory to simulate the molecular dynamics and found that the structure of the solvation shell is as shown in the figure above. In the model, Li+ has a strong interaction with O in TFSI-. After adding NO3- in the solution, NO3- can replace TFSI- (top e) or replace solvent molecules (top graph f) in the solvation shell, thus The effect of weakening the interaction between Li+-solvents is achieved.
At the same time, the above research also shows that the structure of the solvation shell does not necessarily need to maintain electrical neutrality. For example, the above figure e and Figure f show an electronegative structure, which can further interact with Li+ to form a larger structure, thereby further weakening. Li+-solvent force.
The heterogeneous order parameter HOP data also proves the above calculation results. Generally speaking, the greater the order parameter, the stronger the interaction between Li+-solvents, Jun Ming found, 1.0M/0.4M The ordering parameter of the solution is 3.6. When the lithium salt concentration is increased to 2.5M/0.4M, the ordering parameter drops to 1.8, indicating that increasing the lithium salt concentration can effectively reduce the Li+-solvent interaction. .
The ordering parameter of the 2.5M/0M electrolyte without LiNO3 was 4.7, which was much higher than the 1M/0.4M electrolyte with LiNO3 added, indicating that NO3- can effectively reduce the Li+-solvent interaction.
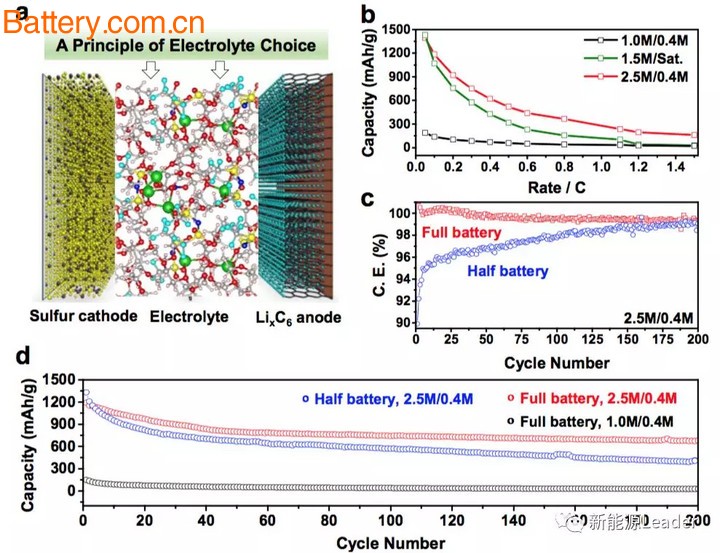
Jun Ming's research has opened up a whole new way of thinking in electrolyte development—reducing Li+-solvent interactions, reducing solvent co-intercalation, and improving the cycle performance of graphite anodes. Reducing the Li+-solvent interaction can be achieved by increasing the concentration of the lithium salt, using a weakly soluble DOL solvent system, adding inorganic salts (such as LiNO3 or NaNO3, etc.) to the electrolyte, thereby significantly reducing solvent co-intercalation. Phenomenon, this provides a new idea for the design of future electrolytes, especially the design of S positive battery electrolyte, which is of great significance for promoting the application of S positive battery.

Written by: å‡
Source: First electric network
Author: New Energy Leader
Rainbow commercial exhaust system is used for boiler and generator in hotel, apartment, hospital, school and date center.
Double wall stainless steel pipe with high quality insulation and corrosion free stainless steel, no thermal bridges between the inner and outer tube.
This exhaust system is easy installation due to perfect fitting plug-in system, no welding on site needed.
Commercial Exhaust System,Commercial Exhaust Fans,Commercial Range Hood,Commercial Kitchen Exhaust Fan
Suzhou Rainbow Environmental Equipment Co., Ltd , https://www.rainbowchimney.com